Materials Selection Guide
An offshore platform can have nearly 50,000 feet of tubing, more than 20,000 fluid system components, no fewer than 10,000 fittings, and as many as 8,000 mechanical connections.
No wonder choosing one material isn’t easy. In fact, there are many considerations when specifying materials for instrumentation lines, hydraulic power, chemical injection, deluge systems, and much more.
That’s where Swagelok can help. We’ve been fighting corrosion since 1947. We simplify selection with our deep understanding of factors that contribute to corrosion, as well as the properties of materials that help fight it. We use alloys with at least two, but often up to ten different elements in optimized concentrations which give our materials superior corrosion resistance that helps our products perform better.
For instance:
Nickel [Ni] + Copper [Cu] = Alloy 400 (Monel®)
Iron [Fe] + Nickel [Ni] + Chromium [Cr] + Molybdenum [Mo] = 316 Austenitic Stainless Steel
With stringent quality control measures, expert-led instruction, and authorized sales and service center support, Swagelok offers unmatched expertise in the world’s toughest environments. We make material selection a matter of confidence for our customers.
> View and download a print-friendly version of the Materials Selection Guide
Featured Content
What is Corrosion?
Just about every metal corrodes under certain conditions.
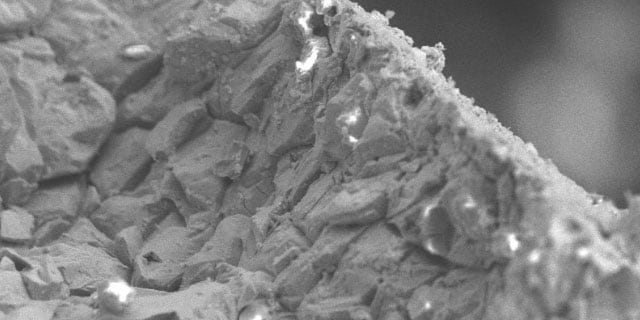
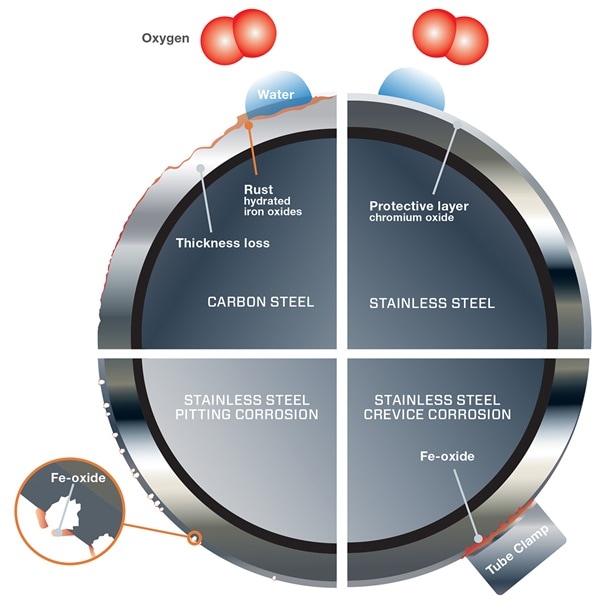
Corrosion is the physical degradation of a material due to interactions with its environment. Corrosion occurs when a metal atom is oxidized by a fluid, leading to a loss of material in the metal surface. This loss reduces the wall thickness of a component and makes it more prone to mechanical failure.
Material Matters
Rust is a commonly occurring byproduct of corrosion, resulting from iron corroding and forming iron oxide. Many other types of corrosion exist, however. Each type poses a threat that must be evaluated when selecting the optimal material for your application.
Take these steps to reduce the impact of corrosion on your applications:
- Identify types of corrosion - what it looks like, where it occurs, and why it happens
- Select materials resistant to corrosion
- Minimize locations where crevice corrosion can occur
- Avoid the contact of dissimilar metals, which can cause galvanic corrosion
- Specify everything from the supports and clamps to the tubing itself to reduce the potential for corrosion
- Understand requirements and standards
- Learn more through training
Identifying Types of Corrosion
Finding a proper materials solution means starting at the source of the problem.

General (Uniform) Corrosion
General, or uniform corrosion, is the easiest to identify. Learn to spot it.

Localized Pitting Corrosion in Chloride-Containing Media
Learn how pitting forms on material surfaces.

Localized Crevice Corrosion in Chloride-Containing Media
See how corrosion can form in a fluid system's crevices and tight spaces.
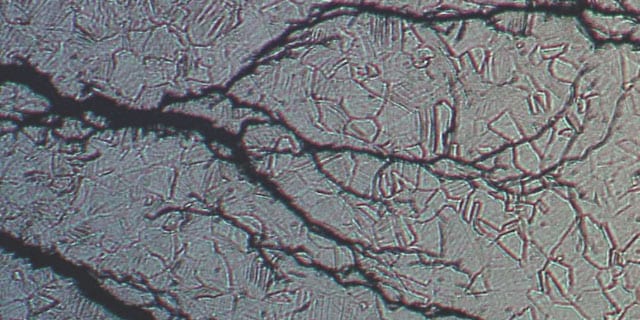
Stress Corrosion Cracking in Chloride-Containing Media
Learn more about how stress corrosion cracking can result in material failure.

Sour Gas Cracking or Sulfide Stress Cracking at High Hydrogen Sulfide (H2S) Partial Pressure
Sour gas environments can lead to sulfide stress corrosion. Discover how it forms.

Hydrogen Embrittlement
Hydrogen atoms can diffuse into metals, making them brittle. Selecting resistant materials can help.

Galvanic Corrosion in the Presence of an Electrolyte
To avoid galvanic corrosion, know what causes it.
Selecting Materials Resistant to Corrosion
Understand the materials available to you that help control the many types of corrosion.
Understanding Requirements and Standards
To ensure quality and consistency, our full range of materials is compliant with metallurgical requirements of NACE and NORSOK standards.
For more information, check out these additional helpful reference materials from Swagelok.
> View and download a print-friendly version of the Materials Selection Guide